
By Kalyan Dhakane, EFFCO Finishes & Technologies Pvt Ltd, and P. P. Deshpande, department of metallurgy and material science, College of  Engineering, University of Pune, India
Engineering, University of Pune, India
Low carbon steels corrode in most atmospheric environments, especially when the relative humidity exceeds 60%. Whilst there are effective coatings used to prevent corrosion, some can contain environmental hazardous pigments.
EFFCO Finishes and Technologies Pvt Ltd recently introduced a non-toxic and environmentally friendly coating formulation under the trade name ECOMET Corundum. 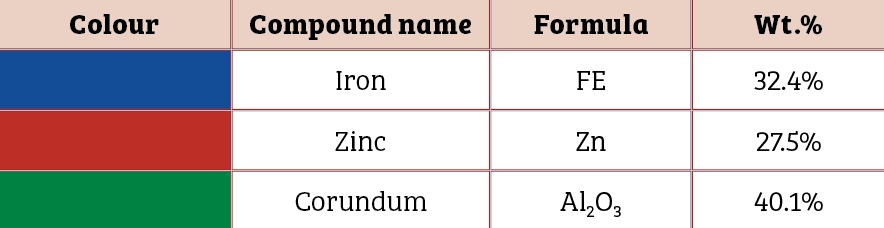 After being applied to steel bolts, the coating displayed no corrosion for 0.5mm and 1mm scratch tests, but did corrode within 1 to 2 days after a 1.5mm scratch test. With that in mind, EFFCO looked to analyse the reasons this corrosion happened within the scratched region.
After being applied to steel bolts, the coating displayed no corrosion for 0.5mm and 1mm scratch tests, but did corrode within 1 to 2 days after a 1.5mm scratch test. With that in mind, EFFCO looked to analyse the reasons this corrosion happened within the scratched region.
Corrosion studies 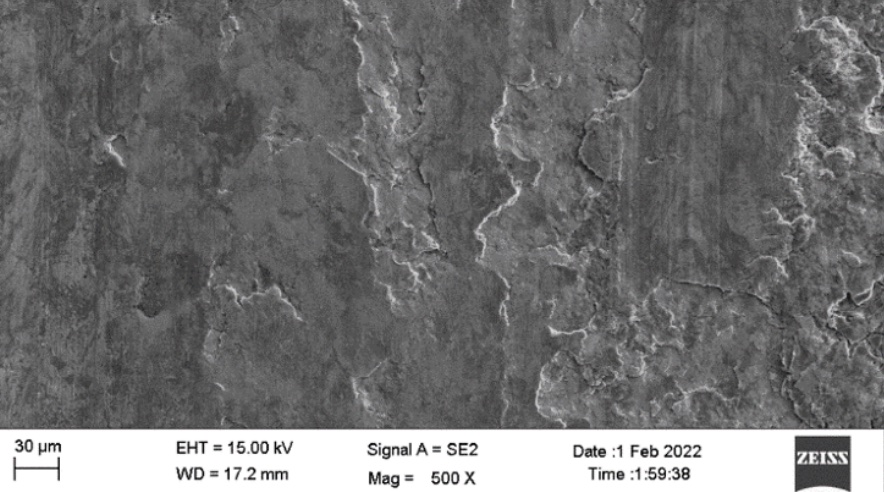
To conduct the test, M10 hex bolts coated with ECOMET Corundum, applied with dip spin method, were used as samples, with the cut made in the bolts with a 500 micron width.
When it came to analysing the bolts, scanning electron microscopy, using field emission scanning electron microscope (FE-SEM, SIGMA IV Carl Zeiss U.K.), was conducted at 3000 X to examine morphology of the coating. X- Ray diffraction (Bruker AXS DS Advance, Germany) was also carri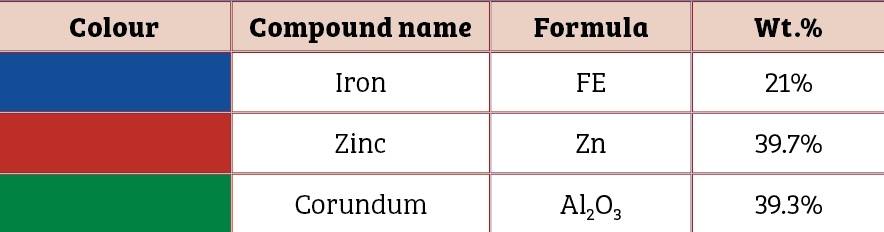 ed out to study the composition of the compound formation at the scratch.
ed out to study the composition of the compound formation at the scratch.
A corrosion cell – having a three electrode geometry of coated steel bolt as working electrode, platinum as counter electrode and saturated calomel electrode (SCE; pH Products, Hyderabad, India) as a reference electrode – was used. The cell related to Gamry reference system 1000 (Wilmington, US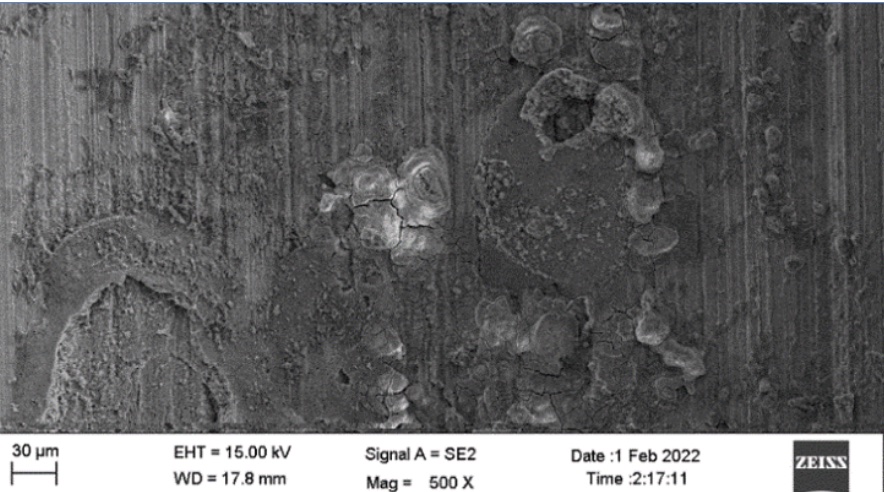 A) for electrochemical measurements.
A) for electrochemical measurements.
Corrosion performance of the coated samples was assessed by electrochemical impedance spectroscopy over frequency range 100 KHz to 0.1 Hz – using amplitude signal 10 mV rms (ASTM G106 and ASTM 2005b). For all the electrochemical experiments, 3.5 wt % NaCl aqueous solution was used. All measurements were carried out five times to obtain good reproducibility of the results, reported below:
Results and discussion 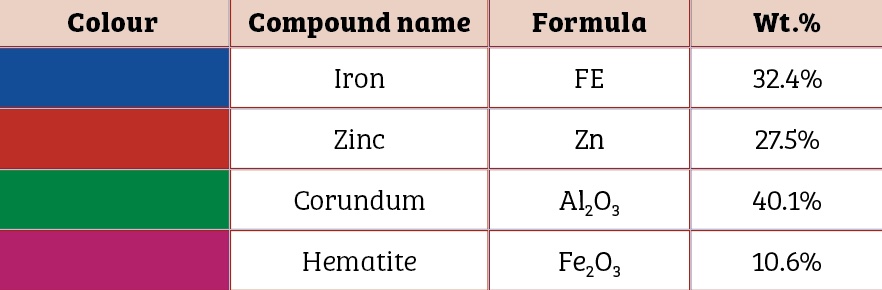
Scanning electron microscopic image of the 0.5mm, 1mm and 1.5mm to 2mm cut sectioned ECOMET Corundum samples are shown in Figures One, Two and Three respectively:
Electro chemical investigation
Table Four contains results of the electrochemical impedance spectroscopic investigations just after immersion and open circuit potential measurements in 3.5 % NaCl.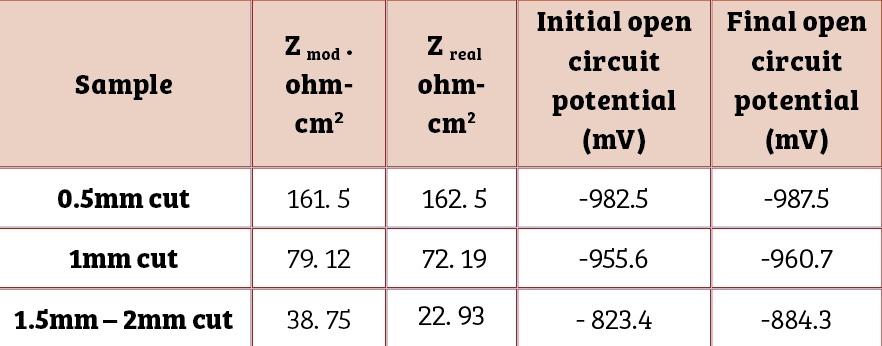
It reveals a decrease in impedance values of the coating with increase in the size of the cut section. This underlines the corrosion tendency of the coated bolt as a function of the scratch size. A negative shift in open circuit potential confirms cathodic protection of the bolt steel.
Corrosion protection mechanism
It is suggested that corundum coating functions by three mechanisms – barrier protection, galvanic protection and the formation of corrosion inhibiting products. The coating, being a barrier, precludes water and oxygen from reaching the metal substrate.
The aluminium oxide imparts dielectric properties to the coating, opposes the passage of corrosive species and improves the barrier action. The barrier mechanism is effective as long as the coating is intact. When the scratch is made, at the cut edges, zinc corrodes preferentially – providing sacrificial protection to the steel.
The resulting product then fills the cavities in the coating and inhibits further corrosion. As the exchange current density of zinc dissolution (10 - 7 A/cm2) is lower than that of the steel (10 - 6 A/cm2), it begins to corrodes first in case of the damage of the coating and it corrodes more slowly than the steel, and thereby protectsthe steel for a longer period. This is the basis of galvanic protection of steel.
It should be noted that zinc is sufficient in surface layer on the bolt to provide electrical contact up to the certain scratch size only. As the scratch size increases beyond critical value, zinc is not adequate to impart sacrificial protection to the base steel (Table Three).
The underlying steel gets exposed due to increased scratch size. Since aluminium is in oxide form, it works as a cathode with respect to the steel. Hence, steel begins to corrode and thereby leads to the formation of an iron oxide i.e. red rust. With that in mind, the coating thickness should be increased to impart corrosion protection to the bolt if the scratch size is 1.5mm to 2mm.

Will joined Fastener + Fixing Magazine in 2007 and over the last 15 years has experienced every facet of the fastener sector - interviewing key figures within the industry and visiting leading companies and exhibitions around the globe.
Will manages the content strategy across all platforms and is the guardian for the high editorial standards that the Magazine is renowned.
Don't have an account? Sign Up
Signing up to Fastener + Fixing Magazine enables you to manage your account details.